2 Mg En Ml
renascent
Sep 20, 2025 · 6 min read
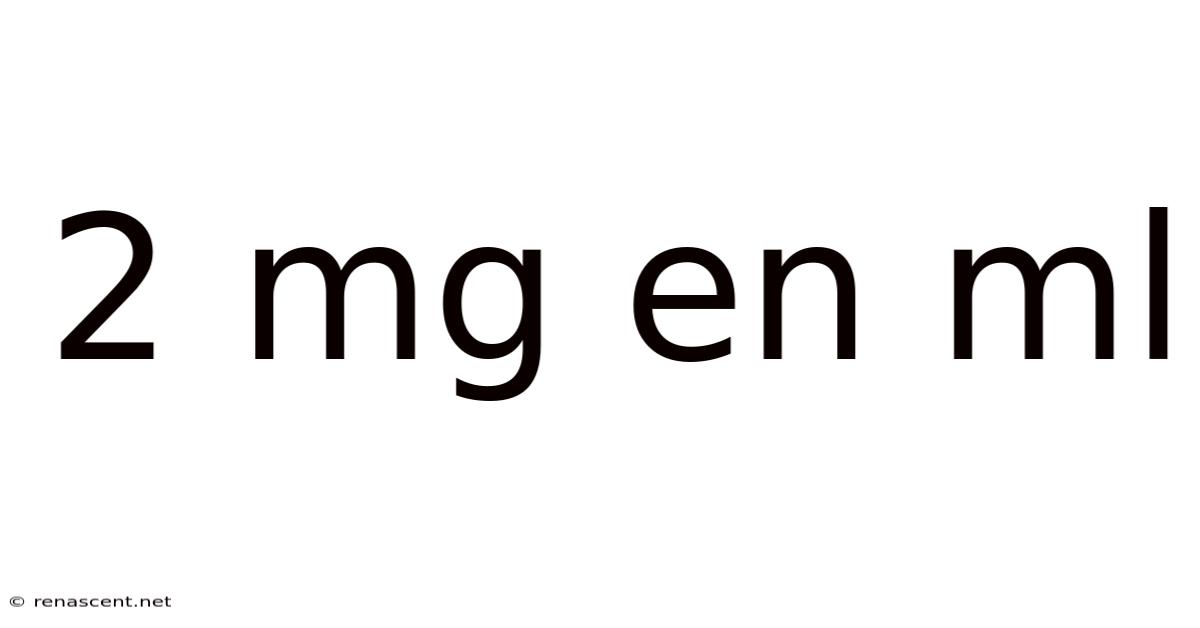
Table of Contents
Understanding 2 mg/mL: A Comprehensive Guide to Concentration and Dosage
Understanding the meaning and implications of "2 mg/mL" is crucial in various fields, from medicine and pharmacy to chemistry and biology. This seemingly simple notation represents a concentration, specifying the amount of a substance dissolved in a given volume of liquid. This article will delve into the intricacies of this concentration unit, exploring its meaning, applications, calculations, and potential pitfalls, ensuring a comprehensive understanding for readers of all backgrounds. We'll cover everything from basic definitions to advanced applications, making this a valuable resource for students, professionals, and anyone curious about this fundamental concept.
What Does 2 mg/mL Mean?
The notation "2 mg/mL" stands for 2 milligrams per milliliter. It expresses the concentration of a solute (the substance being dissolved) within a solvent (the liquid it's dissolved in). In simpler terms, it means that for every milliliter (mL) of the solution, there are 2 milligrams (mg) of the solute present. This is a common unit used to express concentration, particularly in pharmaceutical and biological contexts.
Applications of 2 mg/mL Concentrations
The application of solutions with a concentration of 2 mg/mL is incredibly diverse and spans multiple disciplines. Here are some key examples:
-
Pharmaceuticals: Many medications are administered in solution form, with their concentration precisely defined. A 2 mg/mL concentration might be used for intravenous (IV) infusions, injections, or oral solutions. Accurate concentration is paramount for safe and effective drug delivery. The dosage then becomes dependent on the volume administered.
-
Analytical Chemistry: In laboratories, preparing solutions with specific concentrations is crucial for various analytical techniques. A 2 mg/mL standard solution might be used for calibrating instruments or creating dilution series for quantitative analysis.
-
Biological Research: In cell culture or other biological experiments, researchers often use solutions with specific concentrations of nutrients, drugs, or signaling molecules. A 2 mg/mL concentration of a particular growth factor might be used to stimulate cell growth.
-
Environmental Science: The concentration of pollutants in water or soil samples is often expressed in mg/mL or related units. Monitoring the concentration of contaminants is crucial for assessing environmental health.
Calculating Dosages and Dilutions
Working with concentrations like 2 mg/mL often involves calculating dosages or preparing dilutions. Let's explore these crucial calculations:
Dosage Calculation:
If a patient requires a dose of 4 mg of a medication that has a concentration of 2 mg/mL, how many milliliters should be administered?
The calculation is straightforward:
- Desired dose: 4 mg
- Concentration: 2 mg/mL
- Volume needed: (Desired dose) / (Concentration) = 4 mg / (2 mg/mL) = 2 mL
Therefore, 2 mL of the 2 mg/mL solution should be administered.
Dilution Calculation:
Imagine you need to prepare 100 mL of a 1 mg/mL solution from a stock solution with a concentration of 2 mg/mL. This requires dilution. We can use the formula C1V1 = C2V2, where:
- C1 = initial concentration (2 mg/mL)
- V1 = initial volume (unknown, what we need to find)
- C2 = final concentration (1 mg/mL)
- V2 = final volume (100 mL)
Rearranging the formula to solve for V1:
V1 = (C2V2) / C1 = (1 mg/mL * 100 mL) / 2 mg/mL = 50 mL
Therefore, you need to take 50 mL of the 2 mg/mL stock solution and dilute it with enough solvent to reach a final volume of 100 mL.
Understanding the Importance of Accuracy
Accurate measurement and handling of solutions are critical when working with specific concentrations like 2 mg/mL. Errors can have significant consequences, particularly in medical or research settings.
-
Calibration of Equipment: Using properly calibrated pipettes, volumetric flasks, and balances is paramount for accurate measurements. Regular calibration ensures accuracy.
-
Proper Technique: Following established protocols for solution preparation and handling minimizes errors. This includes proper mixing, avoiding contamination, and ensuring the solution is homogeneous.
-
Significant Figures: Paying attention to significant figures in calculations prevents the propagation of errors and ensures the reported concentration is appropriately precise.
-
Safety Precautions: Always handle solutions with care, wearing appropriate personal protective equipment (PPE) such as gloves and eye protection. Proper disposal of solutions is also crucial.
Potential Pitfalls and Common Mistakes
Several common mistakes can arise when working with concentrations:
-
Unit Confusion: Confusing milligrams (mg) with grams (g) or milliliters (mL) with liters (L) can lead to significant errors in calculations. Always double-check your units.
-
Incorrect Dilution Techniques: Improper dilution techniques, such as inaccurate measurements or incomplete mixing, can result in a solution with the wrong concentration.
-
Ignoring Temperature Effects: Temperature can affect the volume and density of solutions, leading to slight inaccuracies in concentration. Consider this when dealing with precise measurements.
-
Contamination: Contamination can alter the concentration of a solution, affecting experimental results or the efficacy of a medication. Maintaining a clean work environment is essential.
Frequently Asked Questions (FAQ)
Q: Can I convert 2 mg/mL to other concentration units?
A: Yes, you can convert 2 mg/mL to other units like g/L, µg/mL, or ppm (parts per million) using appropriate conversion factors. For example, 2 mg/mL is equal to 2 g/L (since 1 g = 1000 mg and 1 L = 1000 mL).
Q: What is the difference between concentration and dosage?
A: Concentration refers to the amount of solute per unit volume of solution (e.g., 2 mg/mL). Dosage refers to the amount of a substance administered to a patient, which depends on both the concentration and the volume administered.
Q: How do I store a solution with a concentration of 2 mg/mL?
A: Storage conditions depend on the specific solute and its stability. Information about proper storage is typically provided on the label or in the product's documentation. Factors to consider include temperature, light exposure, and container type.
Q: What happens if I use a solution with an incorrect concentration?
A: The consequences depend on the context. In pharmaceutical applications, an incorrect concentration can lead to ineffective treatment or even adverse effects. In research, it can lead to inaccurate or unreliable results.
Conclusion
Understanding the concept of 2 mg/mL, or any concentration for that matter, is fundamental to various scientific and practical fields. Accurate measurement, calculation, and handling are critical to ensure the safe and effective use of solutions with specific concentrations. By understanding the underlying principles and potential pitfalls discussed in this article, individuals can confidently work with these concentrations in a variety of applications, ensuring accuracy and safety in all their endeavors. Always double-check your calculations and procedures to prevent errors, and remember that precision and attention to detail are essential when working with such crucial measurements.
Latest Posts
Latest Posts
-
2 Hours Into Seconds
Sep 20, 2025
-
30 Grams Into Ounces
Sep 20, 2025
-
5 5 Cm Into Mm
Sep 20, 2025
-
Nicaragua V United States
Sep 20, 2025
-
Full Body Safety Harness
Sep 20, 2025
Related Post
Thank you for visiting our website which covers about 2 Mg En Ml . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.