Ch3 Ch2 Ch2 Br
renascent
Sep 17, 2025 · 6 min read
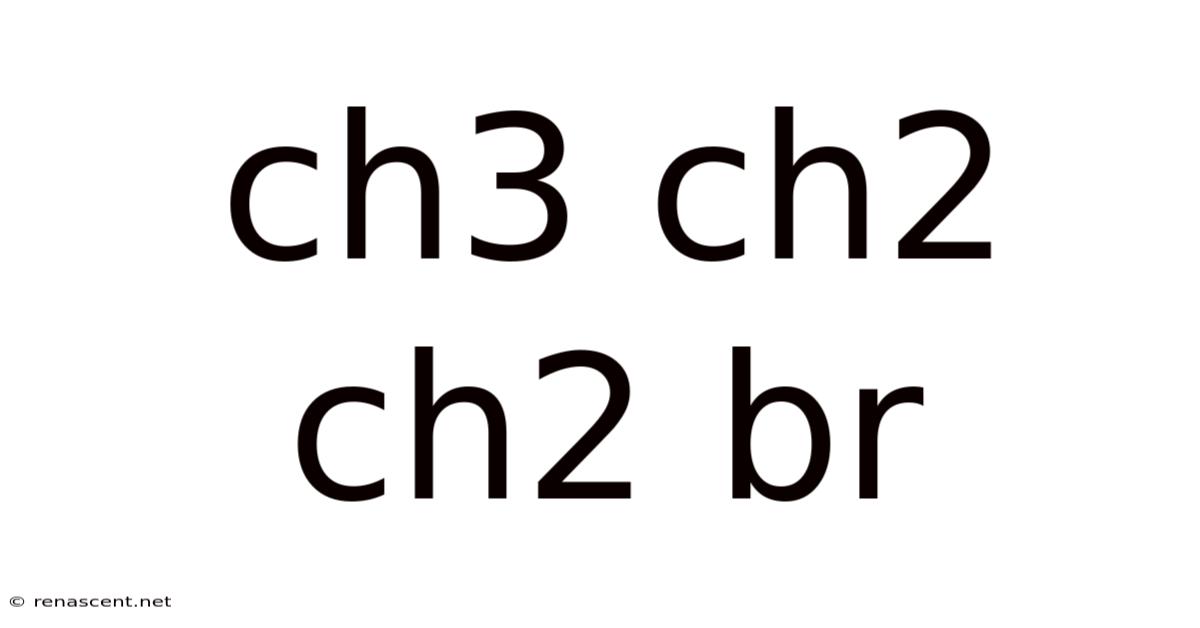
Table of Contents
Understanding 1-Bromopropane: Structure, Properties, Reactions, and Safety
1-Bromopropane, also known as n-propyl bromide, is a chemical compound with the formula CH₃CH₂CH₂Br. This colorless liquid finds applications in various fields, from organic synthesis to its use as a solvent. Understanding its structure, properties, reactions, and safety precautions is crucial for anyone working with this chemical. This comprehensive guide will delve deep into the aspects of 1-bromopropane, providing a thorough overview for students, researchers, and anyone interested in learning more about this important compound.
Introduction: What is 1-Bromopropane?
1-Bromopropane (CH₃CH₂CH₂Br) is an alkyl halide, specifically a primary alkyl halide. This means the bromine atom is attached to a primary carbon atom – a carbon atom bonded to only one other carbon atom. Its structure is relatively simple, consisting of a three-carbon chain with a bromine atom substituted at one end. This seemingly simple structure, however, leads to a range of interesting chemical properties and reactivity. The compound is volatile and possesses a slightly sweet odor, although caution must be exercised as prolonged exposure can be harmful. Understanding its reactivity is key to its safe and effective use in various chemical processes.
Physical and Chemical Properties of 1-Bromopropane
Understanding the physical and chemical properties of 1-bromopropane is essential for its safe handling and use. Let's explore some key characteristics:
- Molecular Weight: Approximately 123 g/mol
- Boiling Point: Around 71 °C (159 °F) - This relatively low boiling point indicates its volatility.
- Melting Point: Approximately -110 °C (-166 °F)
- Density: 1.35 g/cm³ - Slightly denser than water.
- Solubility: Slightly soluble in water, but readily soluble in many organic solvents. This property makes it useful as a solvent in certain organic reactions.
- Appearance: Colorless liquid
- Odor: Slightly sweet odor. Caution: This odor should not be relied upon for detection as the concentration needed to detect the odor might already be hazardous.
Chemical Reactivity of 1-Bromopropane: A Detailed Look
The chemical reactivity of 1-bromopropane stems primarily from the presence of the carbon-bromine bond (C-Br). This bond is polar due to the electronegativity difference between carbon and bromine. This polarity makes the bromine atom a good leaving group in various reactions.
1. Nucleophilic Substitution Reactions (SN1 and SN2): 1-Bromopropane readily undergoes nucleophilic substitution reactions. The mechanism can follow either SN1 (unimolecular nucleophilic substitution) or SN2 (bimolecular nucleophilic substitution) pathways, depending on the reaction conditions and the nucleophile involved.
-
SN2 Reactions: These are favored by strong nucleophiles in polar aprotic solvents. The reaction proceeds in a single step, with the nucleophile attacking the carbon atom bearing the bromine atom from the backside, simultaneously displacing the bromine atom. Examples include reactions with hydroxide ions (OH⁻) to form 1-propanol, or with cyanide ions (CN⁻) to form butyronitrile.
-
SN1 Reactions: These are favored by weak nucleophiles and polar protic solvents. The reaction proceeds through a two-step mechanism involving the formation of a carbocation intermediate. The carbocation is relatively unstable in the case of 1-bromopropane (primary carbocation), making SN1 reactions less favorable than SN2.
2. Elimination Reactions (E1 and E2): Under appropriate conditions, 1-bromopropane can also undergo elimination reactions, resulting in the formation of alkenes.
-
E2 Reactions: These are favored by strong bases at high temperatures. The reaction involves the simultaneous removal of a proton from a beta-carbon (carbon adjacent to the carbon bearing the bromine) and the departure of the bromine atom, leading to the formation of propene.
-
E1 Reactions: Similar to SN1, E1 reactions are less favorable for 1-bromopropane due to the instability of the primary carbocation.
3. Grignard Reagent Formation: 1-Bromopropane can react with magnesium metal in anhydrous diethyl ether to form a Grignard reagent, CH₃CH₂CH₂MgBr. This reagent is a crucial intermediate in many organic synthesis reactions, allowing for the formation of carbon-carbon bonds.
Applications of 1-Bromopropane
1-Bromopropane's reactivity makes it a versatile compound with various applications:
-
Organic Synthesis: It serves as a key building block in the synthesis of many organic compounds, particularly through nucleophilic substitution and Grignard reactions. This allows for the introduction of propyl groups into various molecules.
-
Solvent: Its solubility in organic solvents makes it suitable as a solvent in certain chemical processes. However, due to its toxicity and environmental concerns, its use as a solvent is declining.
-
Intermediate in Pharmaceutical Synthesis: It's used as an intermediate in the synthesis of some pharmaceuticals, although its toxicity necessitates careful handling and controlled usage.
Safety Precautions and Handling of 1-Bromopropane
1-Bromopropane is a hazardous substance requiring careful handling and adherence to safety protocols:
-
Toxicity: 1-Bromopropane is toxic if inhaled, ingested, or absorbed through the skin. It can cause respiratory irritation, central nervous system depression, and other adverse health effects. Prolonged or repeated exposure can be particularly damaging.
-
Flammability: It's a flammable liquid and should be kept away from sources of ignition.
-
Environmental Concerns: 1-Bromopropane is considered an ozone-depleting substance and its release into the atmosphere should be minimized. Many countries have restricted or phased out its use due to environmental regulations.
-
Personal Protective Equipment (PPE): Appropriate PPE, including gloves, safety goggles, and a lab coat, should always be worn when handling 1-bromopropane. Work should be conducted in a well-ventilated area or under a fume hood.
-
Storage: It should be stored in a cool, dry place away from incompatible materials. Proper labeling and storage containers are essential for safe handling.
-
Waste Disposal: Disposal of 1-bromopropane waste should follow local regulations and guidelines. It should not be disposed of down the drain or into the environment.
Frequently Asked Questions (FAQ)
Q: Is 1-bromopropane carcinogenic?
A: While not classified as a definite carcinogen, long-term exposure to 1-bromopropane may pose potential health risks, including potential long-term effects on the nervous system. Therefore, minimizing exposure is crucial.
Q: What are the alternatives to 1-bromopropane in organic synthesis?
A: Several alternative alkylating agents are available, such as 1-iodopropane or other alkyl bromides with less environmental impact. The choice of an alternative depends on the specific reaction and desired outcome.
Q: How is 1-bromopropane produced?
A: One common method for producing 1-bromopropane is through the reaction of 1-propanol with hydrobromic acid (HBr).
Conclusion: A Versatile Compound Requiring Careful Management
1-bromopropane is a valuable compound in organic chemistry, providing a versatile reagent for a range of synthesis reactions. However, its toxicity and environmental impact necessitate careful handling and adherence to strict safety protocols. Understanding its properties, reactivity, and potential hazards is essential for its safe and responsible use in research, industrial settings, and educational environments. As environmental regulations become increasingly stringent, the search for and development of safer alternatives will continue to be a focus in the chemical industry. The information provided here aims to equip readers with a comprehensive understanding of this crucial compound, enabling safe and informed practices while promoting environmental responsibility.
Latest Posts
Latest Posts
-
98 4 Fahrenheit To Celsius
Sep 17, 2025
-
Smaller Than 3 8
Sep 17, 2025
-
7 Years In Days
Sep 17, 2025
-
55 Gallon To Litres
Sep 17, 2025
-
51 60 As A Percentage
Sep 17, 2025
Related Post
Thank you for visiting our website which covers about Ch3 Ch2 Ch2 Br . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.