2 Carbon 4 Hydrogen
renascent
Sep 20, 2025 · 6 min read
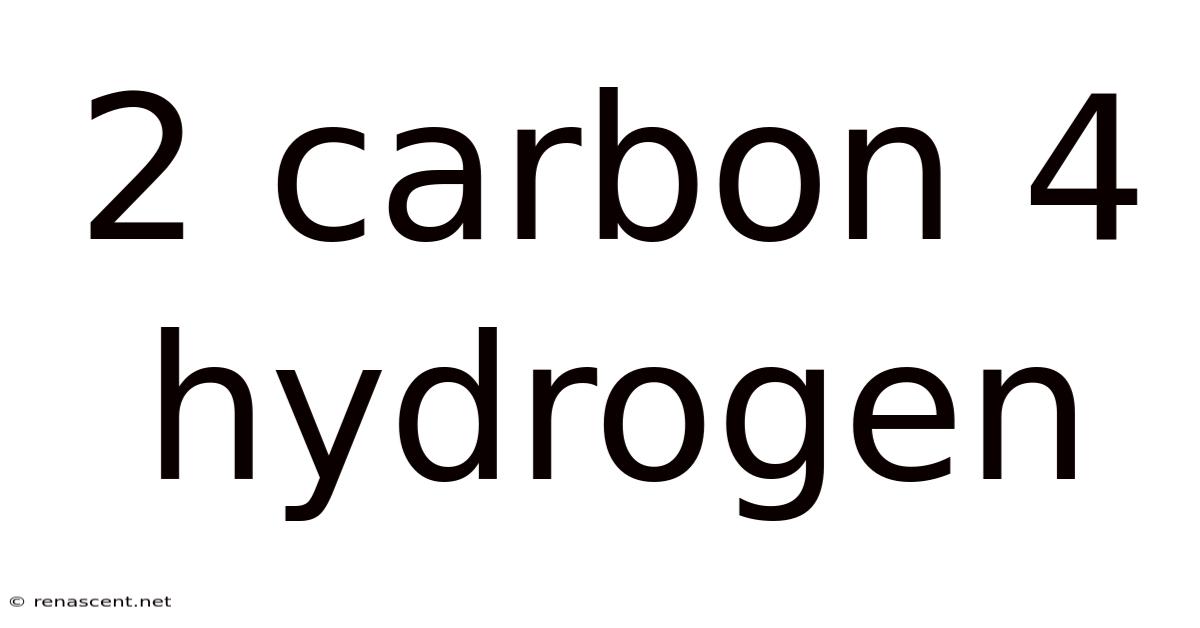
Table of Contents
Decoding the Mystery of C₂H₄: Exploring the World of Ethylene
The chemical formula C₂H₄ represents a molecule of immense significance in the world of chemistry and beyond: ethylene. This simple yet powerful compound, composed of two carbon atoms and four hydrogen atoms, is the foundation of a vast industry and plays a crucial role in various natural processes. This article will delve into the fascinating world of ethylene, exploring its structure, properties, production, uses, and environmental impact. Understanding C₂H₄ opens a window into the intricacies of organic chemistry and its profound influence on our daily lives.
Introduction: A Simple Molecule, a Complex Story
Ethylene (C₂H₄), also known as ethene, is the simplest alkene – a hydrocarbon containing a carbon-carbon double bond. This seemingly small detail is what gives ethylene its unique reactivity and versatility. Its double bond allows it to participate in a wide range of chemical reactions, making it a crucial building block for countless products. From the ripening of fruits to the production of plastics, ethylene's influence is widespread and often unseen. We'll unpack the details of its chemical behavior and explore how these properties translate into its myriad applications.
Understanding the Structure and Bonding of C₂H₄
At the heart of ethylene's unique characteristics lies its molecular structure. The two carbon atoms are connected by a double bond, consisting of one sigma (σ) bond and one pi (π) bond. Each carbon atom also forms single sigma bonds with two hydrogen atoms. This arrangement results in a planar molecule, with all six atoms lying in the same plane. The double bond is shorter and stronger than a single bond, leading to a higher bond energy and influencing its chemical reactivity. This planar structure, with the sp² hybridized carbon atoms, significantly impacts the molecule’s geometry and reactivity. The presence of the pi bond is particularly important, as it is the site of many of ethylene's characteristic reactions.
Production of Ethylene: From Steam Cracking to Industrial Scale
Ethylene is primarily produced through a process called steam cracking. This high-temperature process involves cracking heavier hydrocarbons, such as ethane, propane, butane, and naphtha, in the presence of steam. The high temperatures break down these larger molecules into smaller ones, including ethylene. This process is energy-intensive, but it remains the dominant method for ethylene production due to its efficiency and scale. Other methods, such as the dehydration of ethanol, exist but are less commonly used for industrial-scale production. The optimization of steam cracking processes continues to be a focus of research and development, aimed at improving efficiency and minimizing energy consumption.
Properties of Ethylene: A Versatile Compound
Ethylene exhibits several key properties that contribute to its wide-ranging applications:
- Gas at Room Temperature: Ethylene is a colorless, flammable gas at room temperature and standard pressure. This property dictates its handling and storage requirements.
- Reactivity of the Double Bond: The presence of the carbon-carbon double bond makes ethylene highly reactive. This reactivity allows it to undergo various addition reactions, polymerization, and oxidation reactions.
- Planar Molecular Structure: The planar structure impacts the molecule's interactions with other molecules and influences its reactivity in specific chemical reactions.
- Low Density: Its low density makes it relatively easy to transport and handle compared to other hydrocarbons.
Applications of Ethylene: A Cornerstone of Modern Industry
The versatility of ethylene makes it a crucial building block for a wide range of industries:
- Plastics Production: Ethylene is the primary feedstock for the production of polyethylene (PE), one of the most widely used plastics globally. Different types of polyethylene, such as low-density polyethylene (LDPE) and high-density polyethylene (HDPE), are produced using different polymerization techniques, each with its unique properties and applications. This versatility extends to the creation of various plastic films, bottles, pipes, and many other products.
- Ethylene Oxide Production: Ethylene oxide is a crucial intermediate in the production of various chemicals, including ethylene glycol (used in antifreeze and polyester production) and detergents. It is produced through the direct oxidation of ethylene. The careful control of this reaction is essential to maximize yield and minimize the formation of undesirable byproducts.
- Ethanol Production: Ethylene can be hydrated to produce ethanol, an important solvent and fuel additive. This process involves reacting ethylene with water under specific conditions to yield ethanol.
- Ripening of Fruits: Naturally occurring ethylene plays a crucial role in the ripening of fruits. This process involves complex biochemical pathways, ultimately resulting in the softening and sweetness of the fruit. Commercially, ethylene is used to accelerate the ripening of fruits, allowing for better control over the harvesting and distribution process.
- Other Applications: Ethylene has numerous other applications, including in the production of various chemicals, solvents, and as a refrigerant.
Ethylene and the Environment: Balancing Benefits and Concerns
While ethylene is vital to modern industry, its production and use present environmental concerns:
- Greenhouse Gas Emissions: Ethylene is a greenhouse gas, contributing to climate change. However, its contribution is relatively small compared to other greenhouse gases like carbon dioxide and methane. Continuous improvement in production processes aims to minimize these emissions.
- Air Pollution: Incomplete combustion of ethylene can produce pollutants such as carbon monoxide and particulate matter. Strict regulations and improved combustion technologies aim to minimize such emissions.
- Waste Management: The management of polyethylene waste is a significant challenge. Recycling efforts are crucial to minimize the environmental impact of this widely used plastic.
Safety Precautions: Handling Ethylene Responsibly
Ethylene is a flammable gas and requires careful handling to ensure safety:
- Proper Ventilation: Adequate ventilation is crucial to prevent the buildup of ethylene, which can lead to explosions or asphyxiation.
- Storage: Ethylene should be stored in appropriate containers under controlled conditions to prevent leakage and potential hazards.
- Personal Protective Equipment (PPE): Appropriate PPE, including respiratory protection, should be used when handling ethylene.
Frequently Asked Questions (FAQ)
Q: Is ethylene toxic?
A: Ethylene itself is not highly toxic at low concentrations. However, high concentrations can lead to asphyxiation due to oxygen displacement.
Q: What is the difference between ethylene and polyethylene?
A: Ethylene is a monomer, a single molecule. Polyethylene is a polymer, a large molecule made up of many repeating ethylene units.
Q: Is ethylene found in nature?
A: Yes, ethylene is naturally produced by plants and plays a role in fruit ripening and other plant growth processes.
Q: What are the future prospects for ethylene production?
A: Research and development focus on improving the efficiency and sustainability of ethylene production, including exploring alternative feedstocks and reducing greenhouse gas emissions.
Conclusion: A Vital Compound with Ongoing Significance
Ethylene, with its simple formula C₂H₄, plays a disproportionately large role in modern society. Its unique properties and reactivity make it a cornerstone of various industries, from plastics to agriculture. While environmental concerns associated with its production and use necessitate ongoing efforts towards sustainable practices, the importance of ethylene and its derivatives in our lives remains undeniable. Understanding this remarkable molecule not only illuminates the fascinating world of organic chemistry but also highlights the intricate connections between chemistry, industry, and the environment. Continued research and innovation promise further advancements in its sustainable production and utilization, ensuring its continued significance in shaping the future.
Latest Posts
Latest Posts
-
1 66 Meters To Feet
Sep 20, 2025
-
210 C In F
Sep 20, 2025
-
205 Mins In Hours
Sep 20, 2025
-
Twins From Miss Peregrines
Sep 20, 2025
-
23 25 As A Percentage
Sep 20, 2025
Related Post
Thank you for visiting our website which covers about 2 Carbon 4 Hydrogen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.