3 Ethyl 2 Methylpentane
renascent
Sep 23, 2025 · 6 min read
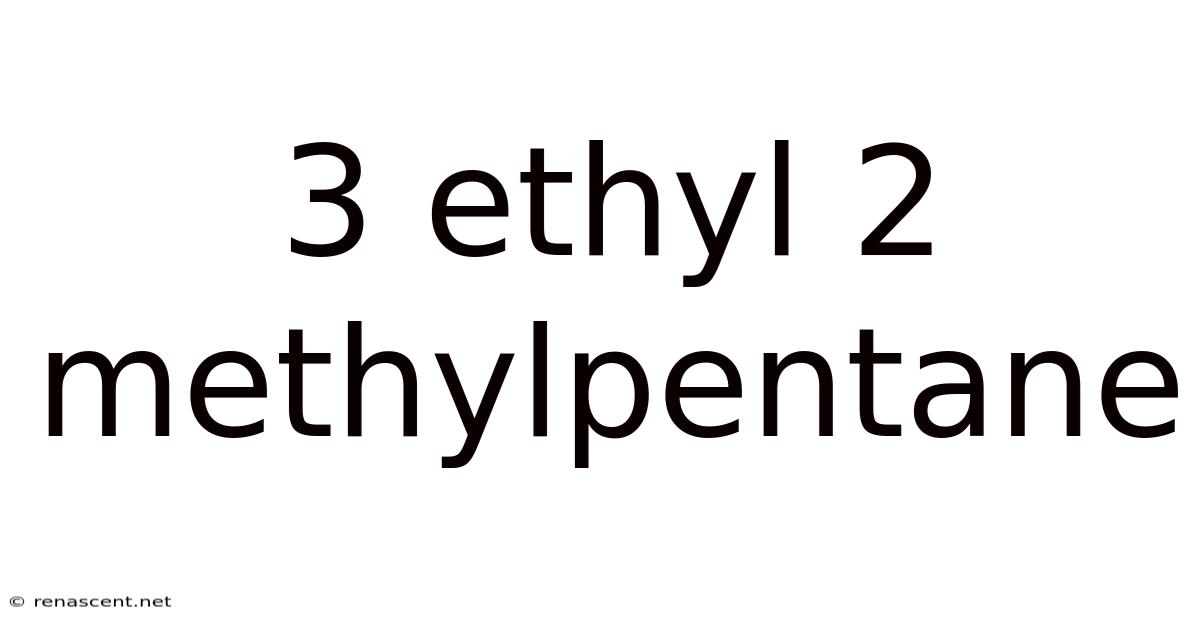
Table of Contents
Decoding 3-Ethyl-2-Methylpentane: A Deep Dive into its Structure, Properties, and Applications
3-Ethyl-2-methylpentane, a seemingly complex name, actually represents a relatively simple branched-chain alkane. Understanding its structure, properties, and potential applications requires a journey into the world of organic chemistry. This article will provide a comprehensive overview, suitable for students and anyone interested in learning more about this specific hydrocarbon. We will explore its nomenclature, physical properties, chemical behavior, and potential uses, aiming to demystify its chemical identity.
Understanding the Nomenclature: Breaking Down the Name
The name "3-ethyl-2-methylpentane" itself reveals its structure. Let's break it down step-by-step:
-
Pentane: This indicates a five-carbon chain as the parent alkane. Think of it as the backbone of the molecule.
-
2-methyl: This signifies a methyl group (CH₃) attached to the second carbon atom of the pentane chain. The numbering starts from the end that gives the substituents the lowest possible numbers.
-
3-ethyl: This tells us that an ethyl group (CH₂CH₃) is attached to the third carbon atom of the pentane chain.
Therefore, the name systematically describes the arrangement of atoms in the molecule. It's crucial to note that the systematic naming, or IUPAC nomenclature, ensures a unique and unambiguous name for every organic compound.
Visualizing the Structure: A 3D Representation
While the name provides valuable information, visualizing the structure is essential for a complete understanding. 3-Ethyl-2-methylpentane has a branched structure, meaning the carbon chain isn't a straight line. We can represent it using various methods:
-
Skeletal Formula: This simplified representation shows only the carbon atoms and their connections, with the understanding that hydrogen atoms are implicitly present to satisfy carbon's four valencies. A skeletal formula would show a five-carbon chain with a methyl group on the second carbon and an ethyl group on the third.
-
Condensed Formula: This representation shows all the atoms, but the bonds are implied. The condensed formula for 3-ethyl-2-methylpentane would be CH₃CH(CH₃)CH(CH₂CH₃)CH₂CH₃.
-
Ball-and-Stick Model: This three-dimensional model uses balls to represent atoms and sticks to represent bonds, providing a more intuitive visualization of the molecule's spatial arrangement.
-
Space-Filling Model: This model shows the relative sizes of the atoms and how they fill space, giving a better representation of the molecule's actual shape and volume.
Physical Properties: Characteristics of 3-Ethyl-2-Methylpentane
Like all alkanes, 3-ethyl-2-methylpentane is a nonpolar molecule due to the relatively equal sharing of electrons between carbon and hydrogen atoms. This nonpolar nature dictates many of its physical properties:
-
State: At room temperature and standard pressure, it exists as a colorless liquid.
-
Boiling Point: Its boiling point is relatively low compared to larger alkanes, reflecting its relatively low molecular weight and weaker intermolecular forces (London dispersion forces). The exact boiling point would need to be determined experimentally, but it would fall within the range typical for similar branched alkanes.
-
Density: It's less dense than water, meaning it would float on water.
-
Solubility: It's insoluble in water due to its nonpolar nature. However, it's miscible with many other nonpolar organic solvents.
-
Flammability: Like all alkanes, it is highly flammable and should be handled with care.
Chemical Properties and Reactivity: Understanding its Behavior
3-Ethyl-2-methylpentane, being a saturated hydrocarbon (alkane), is relatively unreactive under normal conditions. This low reactivity is due to the strong C-C and C-H bonds and the absence of readily available reactive sites like double or triple bonds. However, under specific conditions, it can undergo certain reactions:
-
Combustion: This is the most significant reaction, where it reacts with oxygen to produce carbon dioxide, water, and heat. This reaction is the basis for its use as a fuel.
-
Halogenation: In the presence of ultraviolet light, it can undergo free radical halogenation, where a halogen atom (like chlorine or bromine) replaces a hydrogen atom on the carbon chain. This reaction is not highly selective, leading to a mixture of products.
-
Isomerization: Under specific catalytic conditions and high temperatures, it can undergo isomerization, converting to other isomers with the same molecular formula but different structural arrangements.
Potential Applications: Exploring its Uses
Although not as widely used as some other hydrocarbons, 3-ethyl-2-methylpentane finds applications in several areas, primarily due to its properties as a hydrocarbon:
-
Fuel Component: Its combustion properties make it a potential component in gasoline blends. The branched structure can contribute to improved octane rating, which is crucial for preventing engine knocking.
-
Solvent: Its nonpolar nature and solubility in other organic solvents make it a potential solvent in specific industrial applications, particularly where compatibility with other nonpolar materials is required.
-
Chemical Intermediate: It can serve as a starting material for the synthesis of other organic compounds, although its relatively simple structure might limit the variety of compounds it can readily produce.
Isomers of 3-Ethyl-2-Methylpentane: Exploring Structural Variations
It's important to understand that 3-ethyl-2-methylpentane is just one of many possible isomers with the same molecular formula (C₈H₁₈). Isomers are molecules with the same molecular formula but different structural arrangements. The existence of numerous isomers highlights the complexity and diversity of organic chemistry. Identifying and distinguishing these isomers is a crucial aspect of organic chemistry. Different isomers will have slightly different physical properties (boiling point, density, etc.) and potentially different chemical reactivities.
Frequently Asked Questions (FAQ)
Q: What is the octane rating of 3-ethyl-2-methylpentane?
A: The exact octane rating needs to be determined experimentally and would vary depending on the specific testing method used. However, due to its branched structure, it's likely to have a higher octane rating than straight-chain alkanes of similar molecular weight.
Q: Is 3-ethyl-2-methylpentane toxic?
A: Like many hydrocarbons, it is flammable and its vapors can be irritating. Proper handling and safety precautions are necessary to minimize risks. Long-term exposure or ingestion should be avoided. Consult relevant Safety Data Sheets (SDS) for detailed information.
Q: How is 3-ethyl-2-methylpentane synthesized?
A: The precise synthesis method would depend on the desired purity and scale. It's typically obtained as a component of petroleum fractions and purified through fractional distillation or other separation techniques. Direct synthesis from simpler compounds is less common.
Q: What are the environmental impacts of 3-ethyl-2-methylpentane?
A: As a hydrocarbon, its combustion contributes to greenhouse gas emissions (carbon dioxide). Spills can contaminate soil and water, requiring appropriate cleanup measures. The environmental impact is similar to other alkanes used in fuels.
Conclusion: A Comprehensive Overview
3-Ethyl-2-methylpentane, while perhaps not a household name, represents a fascinating example of a branched-chain alkane. Understanding its structure, properties, and potential applications requires a blend of organic chemistry knowledge and practical considerations. This article aimed to provide a comprehensive overview, demystifying its chemical identity and highlighting its relevance within the broader context of hydrocarbon chemistry and its industrial applications. Further research and experimentation are needed to fully explore its potential uses and refine our understanding of its behavior. This exploration underscores the ongoing need for further investigation and development in the field of organic chemistry and its applications.
Latest Posts
Latest Posts
-
28 In A Fraction
Sep 23, 2025
-
Stress In Japanese Language
Sep 23, 2025
-
Tetra Mono Di Tri
Sep 23, 2025
-
Y And X Words
Sep 23, 2025
-
Atomic Number For Lead
Sep 23, 2025
Related Post
Thank you for visiting our website which covers about 3 Ethyl 2 Methylpentane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.