7.5 Mg Into Ml
renascent
Sep 20, 2025 · 5 min read
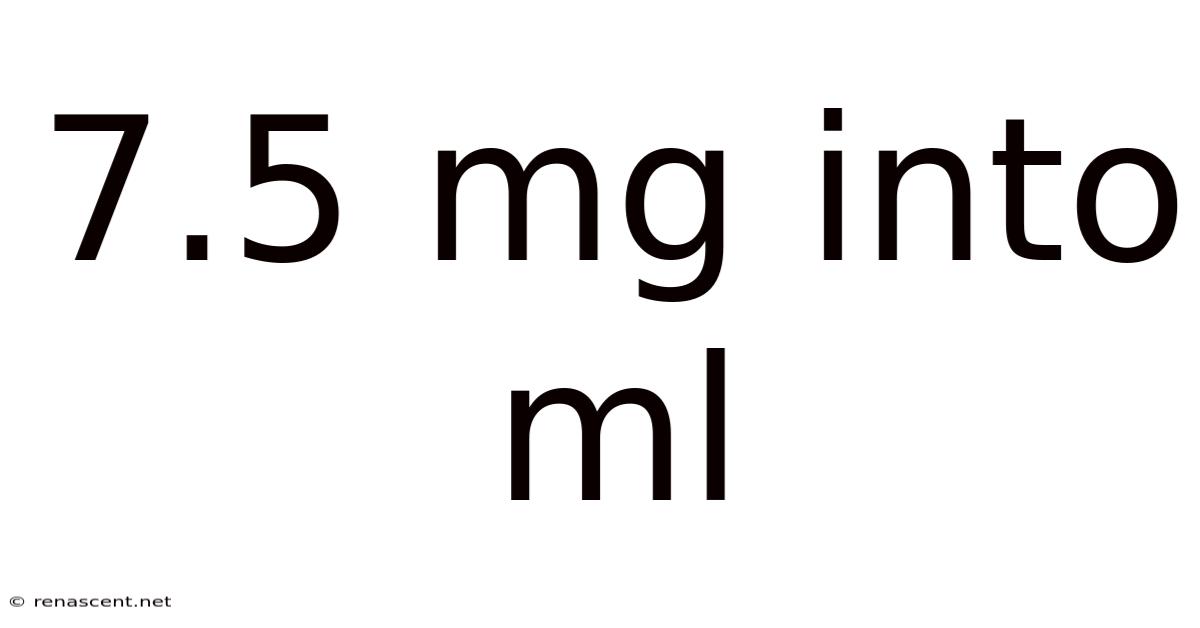
Table of Contents
Converting 7.5 mg into mL: A Comprehensive Guide
Understanding how to convert milligrams (mg) to milliliters (mL) is crucial in various fields, from medicine and pharmacy to chemistry and cooking. This seemingly simple conversion, however, requires careful consideration of the substance involved, as it's not a direct mathematical conversion like converting centimeters to meters. This article provides a comprehensive guide on converting 7.5 mg into mL, explaining the underlying principles and addressing common misconceptions. We'll explore the critical role of density and provide practical examples to solidify your understanding.
Introduction: The Importance of Density
The key to converting milligrams (mg) to milliliters (mL) lies in understanding density. Density is a measure of mass per unit volume. It tells us how much mass a substance has within a given volume. The formula for density is:
Density = Mass / Volume
Therefore, to convert 7.5 mg to mL, we need to know the density of the substance. Without knowing the density, the conversion is impossible. Different substances have vastly different densities. For example, the density of water is approximately 1 g/mL (or 1000 mg/mL), while the density of mercury is significantly higher, around 13.6 g/mL. Attempting to convert 7.5 mg to mL without considering density will yield inaccurate, and potentially dangerous, results.
Understanding Units and Conversions
Before diving into the calculations, let's briefly review the units involved:
- Milligrams (mg): A unit of mass. 1 gram (g) = 1000 mg.
- Milliliters (mL): A unit of volume. 1 liter (L) = 1000 mL.
The conversion from mg to mL is not a direct one. It involves the intermediary step of determining the mass in grams and then utilizing the density to find the volume.
Calculating Volume: Step-by-Step Procedure
Let's assume we have 7.5 mg of a substance with a known density. The procedure for conversion involves the following steps:
-
Convert milligrams to grams: Divide the mass in milligrams by 1000 to obtain the mass in grams.
7.5 mg / 1000 mg/g = 0.0075 g
-
Use the density formula: Rearrange the density formula to solve for volume:
Volume = Mass / Density
-
Substitute values: Substitute the mass (in grams) and the density of the substance into the formula. Let's consider a few examples:
-
Example 1: Water (density ≈ 1 g/mL)
Volume = 0.0075 g / 1 g/mL = 0.0075 mL
-
Example 2: A substance with a density of 2 g/mL
Volume = 0.0075 g / 2 g/mL = 0.00375 mL
-
Example 3: A substance with a density of 0.5 g/mL
Volume = 0.0075 g / 0.5 g/mL = 0.015 mL
-
Practical Applications and Examples
The conversion of milligrams to milliliters is essential in many practical applications. Let's consider some examples:
-
Pharmacology: Many medications are administered in dosages measured in milligrams, but the volume administered might be crucial. For example, a doctor might prescribe 7.5 mg of a specific drug, but the pharmacist needs to know the volume of the solution required, which depends on the drug's concentration and density.
-
Chemistry: In laboratory settings, converting between mass and volume is common. A chemist might need to prepare a solution of a specific concentration, requiring the conversion of a given mass of solute to the necessary volume of solvent.
-
Food Science: In food processing and formulation, accurate measurements are paramount. Converting between mass and volume is important for maintaining consistent recipes and product quality.
-
Environmental Science: When measuring pollutant concentrations in water samples, converting between mass and volume is frequently needed for accurate reporting and analysis.
Dealing with Solutions and Concentrations
When dealing with solutions, an additional factor to consider is the concentration. Concentration expresses the amount of solute (the substance dissolved) in a given amount of solvent (the liquid doing the dissolving) or solution. Common units of concentration include:
- Percent weight/volume (% w/v): Grams of solute per 100 mL of solution.
- Molarity (M): Moles of solute per liter of solution.
If you know the concentration of a solution, you can use it to calculate the volume needed to obtain a specific mass of solute. For example, if you have a 10% w/v solution of a substance and you need 7.5 mg of the substance, you can calculate the required volume as follows:
- Convert 7.5 mg to grams: 0.0075 g
- Set up a proportion: 10 g solute / 100 mL solution = 0.0075 g solute / x mL solution
- Solve for x: x = (0.0075 g * 100 mL) / 10 g = 0.075 mL
Frequently Asked Questions (FAQ)
-
Q: Can I always convert mg to mL directly?
- A: No. You need to know the density of the substance. The conversion is not a straightforward unit conversion.
-
Q: What if I don't know the density of the substance?
- A: You cannot accurately convert mg to mL without knowing the density. You may need to consult a reference source or perform an experiment to determine the density.
-
Q: Are there online calculators for this conversion?
- A: While some online calculators might exist, it's crucial to ensure the accuracy and reliability of the calculator and to always double-check your results. Understanding the underlying principles is more important than relying solely on a calculator.
-
Q: Is it always safe to assume a density of 1 g/mL?
- A: No. Only assume a density of 1 g/mL if you are dealing with water at standard temperature and pressure. Most substances will have different densities.
Conclusion: Accuracy and Precision
Converting 7.5 mg to mL is not a simple calculation; it necessitates understanding the concept of density and the substance's specific density. While the mathematical steps are straightforward, the importance of accuracy cannot be overstated, particularly in fields like medicine and chemistry where precise measurements are critical for safety and efficacy. Remember, always consider the density of the substance before attempting any conversion between mass and volume. This guide provides a solid foundation for understanding and performing these essential conversions accurately and safely. Always double-check your calculations and refer to reliable sources for density information. By mastering this conversion, you'll enhance your understanding of fundamental scientific principles and improve your proficiency in various practical applications.
Latest Posts
Latest Posts
-
Beverage Holder For Car
Sep 20, 2025
-
325 Kpa To Psi
Sep 20, 2025
-
18 Percent Of 120
Sep 20, 2025
-
Pink And Purple Mixed
Sep 20, 2025
-
Schrodinger Equation Time Dependent
Sep 20, 2025
Related Post
Thank you for visiting our website which covers about 7.5 Mg Into Ml . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.