Ch3 C O H
renascent
Sep 16, 2025 · 7 min read
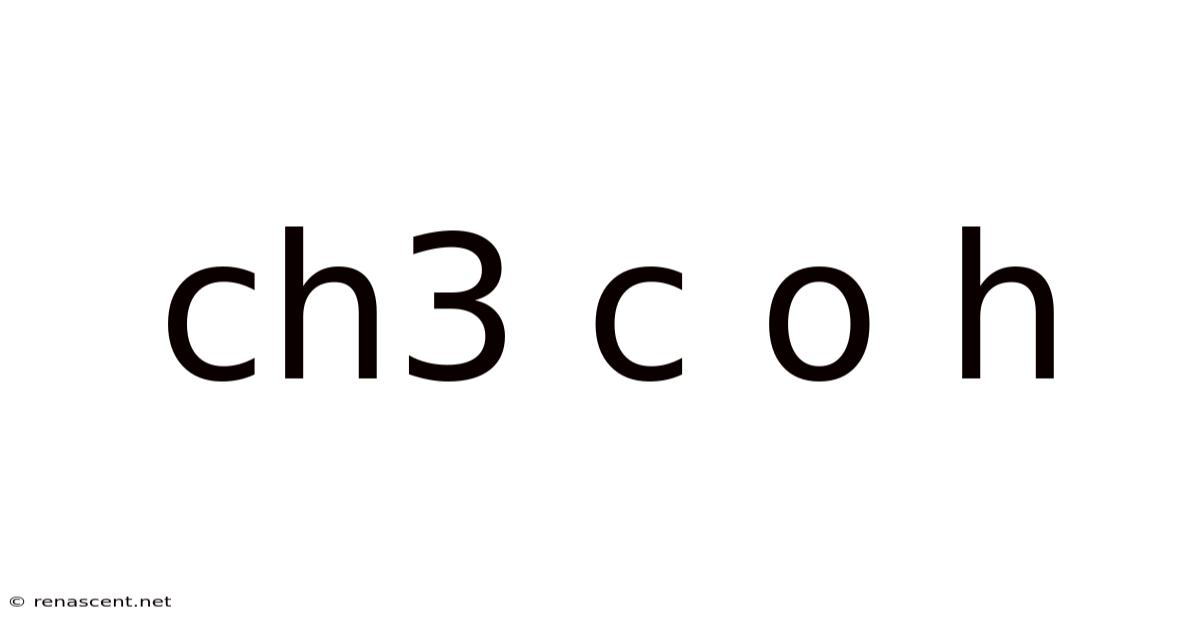
Table of Contents
Unveiling the Mysteries of CH3COH: Acetaldehyde and its Significance
Acetaldehyde, with its chemical formula CH3CHO (often simplified to CH3COH), is a fascinating molecule with a surprisingly significant role in various fields, from industrial chemistry to biological processes. This comprehensive guide will delve into the intricacies of acetaldehyde, exploring its properties, production methods, applications, and biological implications. Understanding acetaldehyde offers a window into the broader world of organic chemistry and its impact on our lives.
Introduction to Acetaldehyde (CH3CHO)
Acetaldehyde, also known as ethanal, is the simplest aldehyde. It's a volatile, colorless liquid with a pungent, fruity odor. This characteristic smell is often described as similar to green apples or burnt almonds, and its presence can be detected even at low concentrations. Its relatively simple structure belies its complex chemistry and wide-ranging applications. This article will equip you with a comprehensive understanding of acetaldehyde, covering its physical and chemical properties, production methods, industrial uses, biological roles, and safety considerations.
Physical and Chemical Properties of Acetaldehyde
Understanding the physical and chemical properties of acetaldehyde is crucial to its safe handling and application. Let's explore these key characteristics:
- Physical State and Appearance: At room temperature, acetaldehyde exists as a colorless liquid.
- Odor: It possesses a characteristic pungent, fruity odor, often described as similar to green apples or slightly acrid.
- Boiling Point: Acetaldehyde has a relatively low boiling point of 20.2 °C (68.4 °F), making it highly volatile.
- Solubility: It is highly soluble in water, ethanol, and ether, demonstrating its polar nature.
- Reactivity: Acetaldehyde's aldehyde functional group (-CHO) makes it highly reactive. It readily undergoes oxidation to form acetic acid (CH3COOH) and reduction to form ethanol (CH3CH2OH). It also participates in various condensation reactions, forming more complex molecules.
- Flammability: Acetaldehyde is highly flammable and its vapors can form explosive mixtures with air.
Production Methods of Acetaldehyde
Several methods are employed for the industrial-scale production of acetaldehyde. The choice of method depends on factors like cost-effectiveness and the availability of raw materials. Here are some of the most common methods:
- Wacker Process: This is currently the most dominant industrial method. It involves the oxidation of ethylene (C2H4) using a palladium(II) chloride catalyst in an aqueous solution. The process is highly efficient and produces high yields of acetaldehyde.
- Oxidation of Ethanol: Ethanol (ethyl alcohol) can be oxidized to acetaldehyde using various oxidizing agents, including potassium dichromate (K2Cr2O7) or potassium permanganate (KMnO4). This method is less common for large-scale production due to lower efficiency and cost.
- Hydroformylation of Methanol: This method involves the reaction of methanol (CH3OH) with carbon monoxide (CO) and hydrogen (H2) in the presence of a catalyst, typically a cobalt or rhodium complex. This process is less common for acetaldehyde production but is significant for producing other aldehydes.
Applications of Acetaldehyde
Acetaldehyde's versatile reactivity makes it a crucial intermediate in the synthesis of numerous chemicals. Its applications span several industries:
- Production of Acetic Acid: Acetaldehyde is a key precursor in the manufacturing of acetic acid (CH3COOH), a vital chemical used in the production of vinegar, plastics, and solvents.
- Production of Pyridine and Pyridine Derivatives: Acetaldehyde plays a crucial role in the synthesis of pyridine and its derivatives, which are valuable compounds used in pharmaceuticals, pesticides, and other industrial applications.
- Synthesis of Other Aldehydes and Ketones: Acetaldehyde serves as a building block for the synthesis of various aldehydes and ketones through reactions like aldol condensation.
- Solvent and Intermediate in Chemical Synthesis: Its solubility and reactivity make it a valuable solvent and intermediate in various organic syntheses.
- Production of Resins: Acetaldehyde contributes to the production of certain types of resins and polymers.
- Food Industry (limited use): In extremely controlled amounts, it can be used as a flavoring agent, primarily for its contribution to specific flavor profiles. However, this use is highly regulated due to its toxicity.
Biological Significance and Metabolism of Acetaldehyde
Acetaldehyde plays a crucial, albeit often negative, role in biological systems. Its presence is significant in both beneficial and detrimental contexts:
- Ethanol Metabolism: The primary source of acetaldehyde in the human body is the metabolism of ethanol (alcohol). Alcohol dehydrogenase (ADH) enzymes catalyze the oxidation of ethanol to acetaldehyde in the liver. This is the first step in the breakdown of alcohol, and the accumulation of acetaldehyde contributes to the symptoms of alcohol intoxication, including nausea, headache, and flushing.
- Acetaldehyde Dehydrogenase (ALDH): The next crucial enzyme in alcohol metabolism is acetaldehyde dehydrogenase (ALDH), which converts acetaldehyde into acetic acid, a less toxic compound. Genetic variations in ALDH activity can affect the rate of acetaldehyde metabolism, leading to increased sensitivity to alcohol and the development of adverse reactions.
- Role in Certain Metabolic Pathways: Although primarily known for its role in alcohol metabolism, acetaldehyde is also involved in some other metabolic pathways, though less prominently.
- Reactive Oxygen Species (ROS) Production: Acetaldehyde can contribute to the formation of reactive oxygen species (ROS), which are harmful molecules that can damage cells and contribute to oxidative stress. This contributes to various health problems associated with alcohol consumption.
Health Effects and Safety Considerations
Acetaldehyde's reactivity and potential for toxicity necessitate careful handling and safety precautions. Exposure to acetaldehyde can lead to various health problems:
- Acute Exposure: Short-term exposure to high concentrations of acetaldehyde can cause irritation of the eyes, nose, and throat, as well as headaches and nausea. High levels can also lead to more severe effects, including respiratory problems and central nervous system depression.
- Chronic Exposure: Long-term exposure to lower concentrations of acetaldehyde has been linked to increased risks of cancer, particularly cancers of the respiratory tract and liver.
- Carcinogen: Acetaldehyde is classified as a human carcinogen (cancer-causing agent) by several international organizations.
- Occupational Exposure: Workers in industries that use or produce acetaldehyde need to take appropriate precautions to minimize exposure, including the use of personal protective equipment (PPE) such as respirators and gloves.
- Environmental Concerns: Acetaldehyde is also a common pollutant in the environment, released through industrial emissions and vehicle exhaust.
Frequently Asked Questions (FAQ)
Q: Is acetaldehyde found naturally?
A: Yes, acetaldehyde occurs naturally in small amounts in various fruits, vegetables, and coffee. However, the concentrations are generally much lower than those found in industrial settings or as a result of alcohol metabolism.
Q: What is the difference between acetaldehyde and acetic acid?
A: Acetaldehyde (CH3CHO) is an aldehyde, while acetic acid (CH3COOH) is a carboxylic acid. Acetic acid is the product of the oxidation of acetaldehyde. The key difference lies in the functional group: -CHO in acetaldehyde and -COOH in acetic acid.
Q: How is acetaldehyde removed from the body?
A: The primary pathway for acetaldehyde removal is through its conversion to acetic acid by the enzyme acetaldehyde dehydrogenase (ALDH). Acetic acid is then further metabolized and excreted from the body.
Q: What are the symptoms of acetaldehyde poisoning?
A: Symptoms of acetaldehyde poisoning can range from mild irritation of the eyes, nose, and throat to severe effects, including respiratory problems, central nervous system depression, and even death in extreme cases.
Q: Is acetaldehyde used in food?
A: While it's present naturally in some foods, its direct addition as a food additive is extremely limited and heavily regulated due to its toxicity. It may be found in trace amounts in certain products as a by-product of other processes.
Conclusion
Acetaldehyde, while a simple molecule, plays a multifaceted role in various aspects of chemistry and biology. Its significance in industrial processes, as a metabolic intermediate, and its potential health hazards highlight the importance of understanding its properties and handling it safely. Further research into its biological effects and potential applications continues to reveal new insights into this fascinating and versatile chemical. The information provided here offers a comprehensive overview, providing a foundation for further exploration of this significant compound. From its industrial applications to its role in alcohol metabolism, acetaldehyde stands as a powerful example of the complexities inherent in even seemingly simple chemical structures. Remember, always prioritize safety when handling this volatile and potentially harmful substance.
Latest Posts
Latest Posts
-
960 Kj To Calories
Sep 17, 2025
-
200 Km Into Miles
Sep 17, 2025
-
4 25 Square Root
Sep 17, 2025
-
Funeral Oration By Pericles
Sep 17, 2025
-
Area Of A Park
Sep 17, 2025
Related Post
Thank you for visiting our website which covers about Ch3 C O H . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.